Current location: Home > NEWS > Industry news
NEWS
PRODUCTS
Extended Application of Molecular Classification of Endometrial Cancer (Ⅰ)
News source: Release time:[2025-11-13]
Background
Ovarian endometrioid cancer (OEC) is an epithelial malignant tumor of the ovary, similar to primary endometrial cancer in both origin and histopathology. Its incidence ranks second only to high-grade serous cancer of the ovary, accounting for approximately 10% of epithelial malignant tumors of the ovary [1].
From a pathogenesis standpoint, there are two major hypotheses. One posits a malignant transformation originating from ectopic endometrium, while the other suggests a classical pathway originating from the germinal epithelium of the ovary. The occurrence of OEC is closely related to endometriosis (EMs): approximately 30% to 50% of OEC patients also have ovarian endometriosis cysts, and the cancer cells are a result of malignant transformation from ectopic endometrial tissues. Consequently, EMs is considered a precursor lesion of OEC. The increased risk of EMs developing into OEC elevates the risk of OEC by 2.32 times. Other studies have indicated that extra-ovarian EMs can also lead to malignant transformation, with 80% being endometrial-like cancer subtypes [2].
In terms of incidence and prognosis, OEC is more prevalent in perimenopausal women, with an average onset age of around 50 years, which is slightly lower than that of high-grade serous cancer. The 5-year survival rate for patients with OEC exceeds 80%. For patients with stage I, the survival rate is 95%; stage II, 84%; stage III, 59%; and stage IV, 29%. The survival rate for OEC is higher than that of other types of epithelial ovarian cancer, including serous, mucinous, and clear cell types [3]. The clinical-pathological stage and histological grade of the tumor are independent prognostic factors.
Molecular characteristics
Whole exon group sequencing reveals that the common molecular mutations in OEC encompass CTNNB1, PTEN gene mutations, and microsatellite instability (MSI). Additionally, mutations are observed in dMMR or POLE (15%), HRD (19%), BRCA1/2 (6%), and PIK3CA (31%) [4].
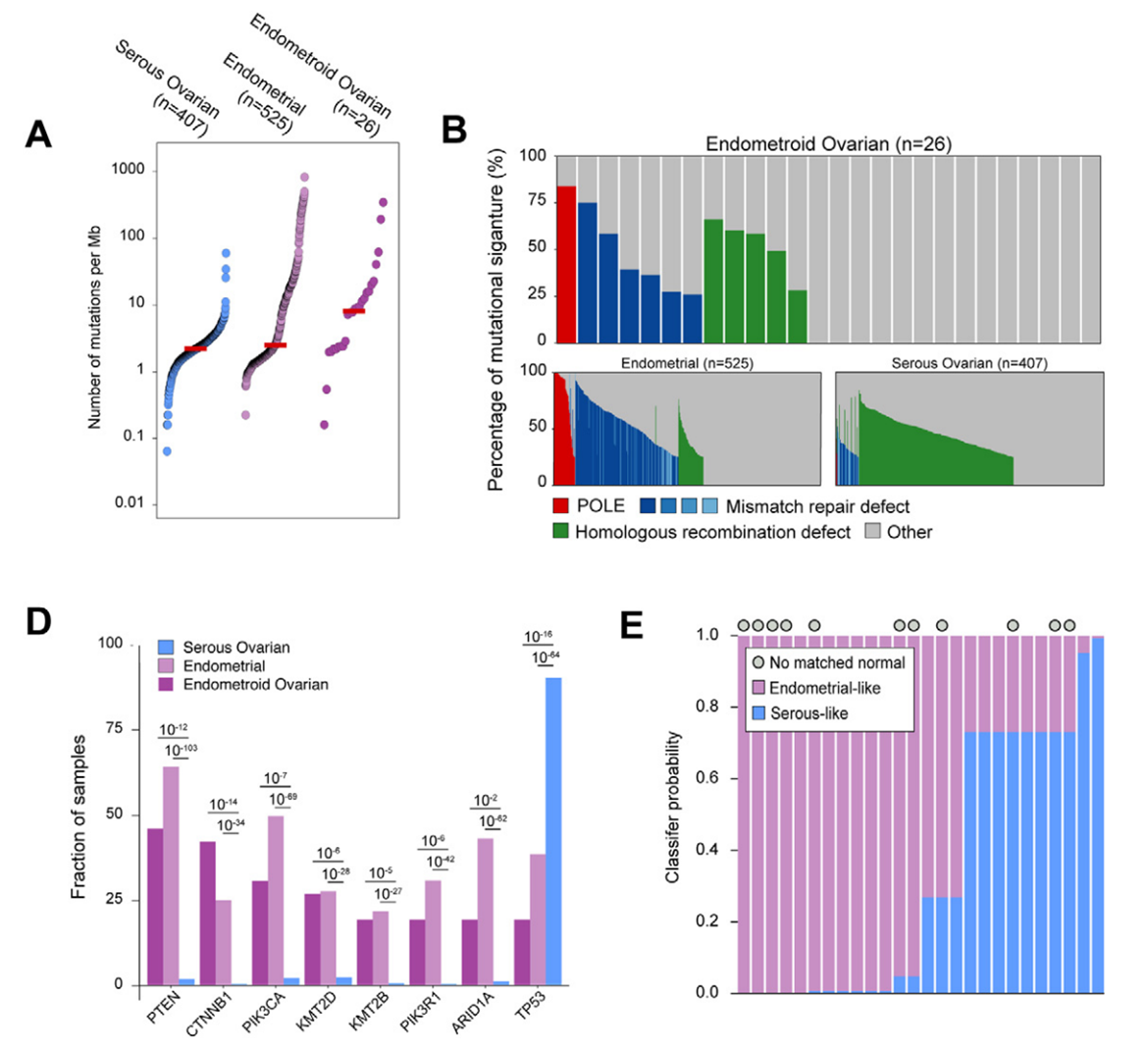
Gene analysis of different histology
Approximately 15% of ovarian cancer cases are linked to germ cell gene mutations, with the BRCA1 and BRCA2 gene alterations being the most prevalent. Nevertheless, BRCA1/2 mutations are infrequent in endometrioid ovarian cancer, occurring in only 5% to 10% of cases, and are predominantly observed in high-grade serous cancers. The TP53 gene mutation is prevalent in high-grade serous cancer and is found in about 25% of endometrioid ovarian cancers, correlating with increased genomic instability.
Application of molecular classification
Due to the similarities in histopathological and molecular features between ovarian endometrioid cancer and endometrial endometrioid cancer, the EC classification scheme can be extended to the latter. A study demonstrated that the proportions of each TCGA molecular subtype in ovarian endometrioid cancer and endometrial endometrioid cancer were as follows: the proportions of the POLE mutant were 5% and 7.6%, respectively (P = 0.594); the percentages of defect types with mismatch repair were 14.6% and 29.2% (P < 0.001), respectively; the percentages of abnormal p53 were 14% and 7.8% (P = 0.097), respectively; and the percentages of nonspecific molecular patterns were 66.4% and 55.4%, respectively (P = 0.002). Progression-free survival analyses are presented in the following table.
Although there are differences in the proportion and pathological features of each TCGA molecular subtype in ovarian endometrioid cancer and endometrial endometrioid cancer, the prognostic values of the TCGA molecular subtypes in the two cancers are similar.
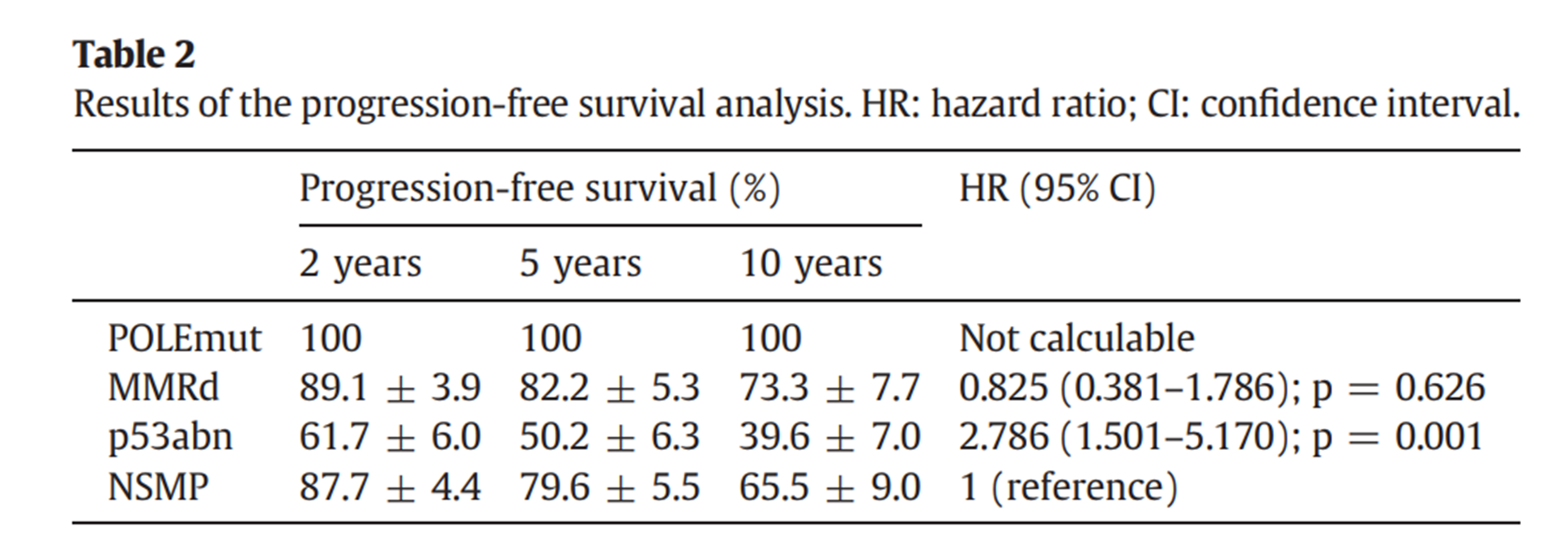
PFS analysis of different subtypes [5]
Another multicenter retrospective cohort study, conducted across multiple hospitals with a relatively large cohort size and an extended follow-up period, was performed. Additionally, a gynecologic pathologist independently reviewed the histology and immunohistochemical staining results, and a joint review of the divergent cases was conducted. As a result, out of the 167 patients enrolled, 1.2% had POLEmut type tumors, 6.6% had MMRd type tumors, 11.4% had p53abn type tumors, and 80.8% had NSMP type tumors. No ER-negative tumors were identified in the POLEmut and MMRd subtypes, and ER status lacked prognostic value in the p53abn subtypes. Within the NSMP subtypes, 11.1% of the tumors were ER-negative, and the 10-year overall survival rate was lower (HR=3.92, 95% CI=1.67-9.21, p=0.002)[6].
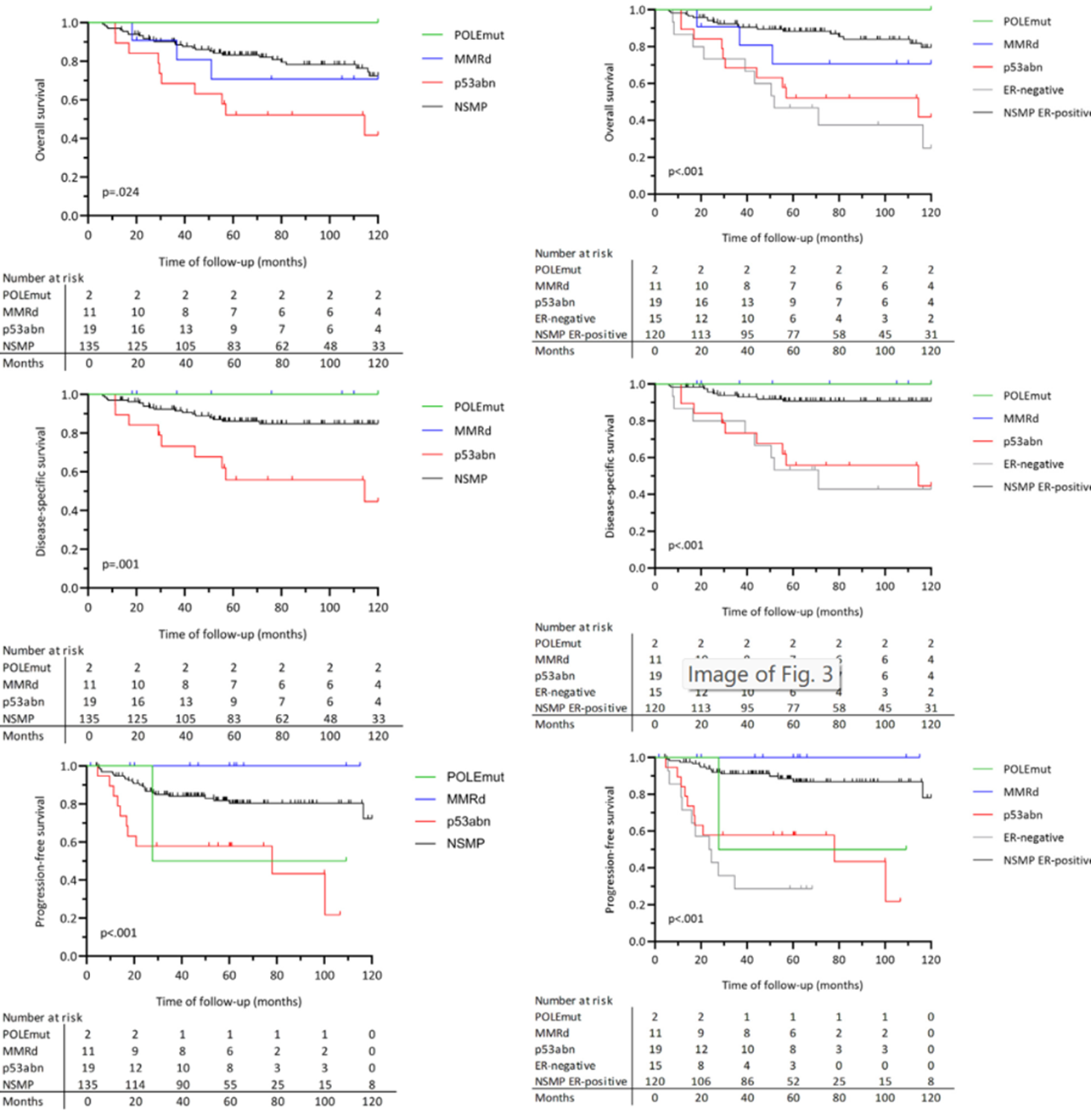
Analysis of research results [6]
Treatment recommendations
The NCCN guidelines recommend that patients with G1 ovarian cancer undergo observation if they are in stage IA/IB. For patients in stage IC, the guidelines suggest either observation or chemotherapy. For those in stages II-IV, systemic adjuvant chemotherapy or hormone therapy should be considered post-surgery. Additionally, the guidelines note that MSI/MMR testing is recommended for all patients with ovarian endometrioid cancer, which has the highest proportion of ovarian tumors with mismatch repair defects (MMRd) associated with Lynch syndrome (with an incidence of MMRd in this subtype ranging from 7% to 10%). MMRd is rare in other histological subtypes. Consequently, this testing can help identify cases of Lynch syndrome in ovarian cancer patients and provide a basis for selecting the appropriate population for immune checkpoint inhibitor treatment.
Treatment recommendations for patients with G2/3 are consistent with those for high-grade serous ovarian cancer (HGSOC), with postoperative chemotherapy being the first-line treatment. For platinum-sensitive patients in stages II-IV, PARP inhibitor maintenance therapy is considered after first-line chemotherapy.
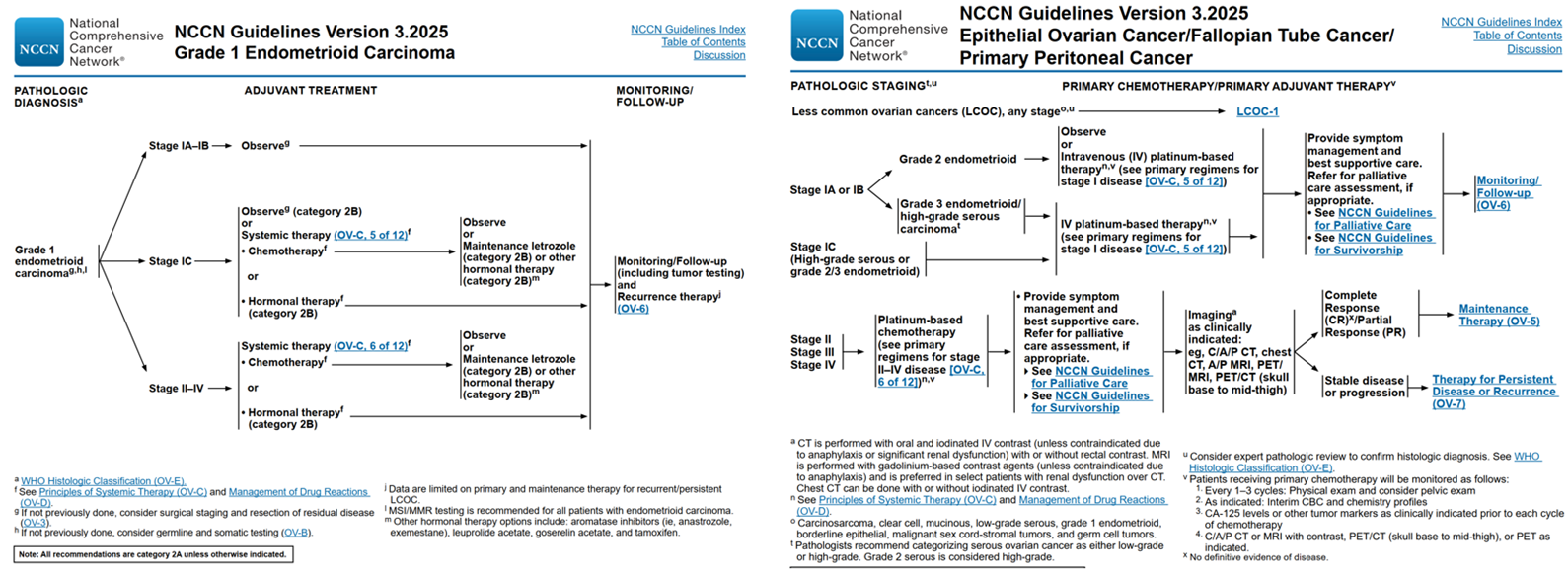
NCCN Guide Recommendations [7]
The expanded application of endometrial molecular classification to ovarian endometrioid cancer-like cancer represents not only a technical breakthrough but also an innovation in the diagnosis and treatment concept. It allows us to shift our focus from "tumor location" to "tumor molecular characteristics" and from "uniform treatment" to "personalized treatment plans."
For patients with ovarian endometrioid cancer (OEC), this signifies increased hope for survival: early-stage patients can avoid overtreatment, late-stage patients can find a precise treatment direction, and younger patients can maintain their fertility. For clinicians, this offers a clearer diagnostic and treatment pathway, with each treatment decision backed by "molecular evidence."
Nevertheless, there are still unavoidable issues that cannot be overlooked. Most current studies are retrospective, and there is a scarcity of prospective independent cohort studies. It is hoped that future research will emerge to provide additional clinical evidence supporting the application of molecular classification in patients with OEC.
References
[1] CA Cancer J Clin. 2018 Jul; 68(4):284-296.
[2] JAMA. 2025 Jul 1; 334(1):64-78.
[3] China Experts Consensus on the Clinical Diagnosis and Treatment of Ovarian Endometrioid cancer (2023 Version)
[4] Gynecol Oncol. 2020 Apr; 157(1):55-61.
[5] Gynecol Oncol. 2021 Nov; 163(2):427-432 ..
[6] Gynecol Oncol. 2025 May:196:137-145.
[7] NCCN ovarian cancer diagnosis and treatment guidelines 2025.v3
Statement: This article is only for sharing, if it involves copyright issues, please contact us as soon as possible, we will correct the first time, thank you!