Current location: Home > NEWS > Industry news
NEWS
PRODUCTS
New Thoughts on Targeted Therapy for Lung Cancer: Can the "Subtraction" Strategy Achieve a Win-Win of Efficacy and Quality of Life?
News source: Release time:[2025-11-13]
With the recent launch of the FDA's OPTI MUS program, cancer treatment is moving from a "one size fits all" model to a new era of personalized medicine. Doctors began to ask not "what is the maximum dose", but "what is the minimum effective dose". In the next five years, we will see more dynamically adjusted treatment options based on blood concentration monitoring and gene testing, so that patients with lung cancer can truly achieve high-quality long-term survival while maintaining efficacy.
For patients with non-small cell lung cancer (NSCLC) carrying specific gene mutations (such as EGFR, ALK, and so on), the emergence of Targeted Therapies, TT) is undoubtedly a revolution. These precise anti-cancer drugs have significantly improved the survival conditions of patients and have become the standard treatment plan at all stages[1,2]. However, with the widespread use of targeted agents, new questions have arisen: have we "overtreated" our patients?
At present, the dose and treatment course of targeted drugs are mostly determined based on early clinical trials, and the core logic comes from traditional chemotherapy–the larger the dose, the better the efficacy. However, the action mechanism of targeted drugs is completely different from chemotherapy. Higher dose often only brings more serious toxic and side effects, but the efficacy is not necessarily in direct proportion. In addition, high drug costs and long-term treatment place a heavy economic burden on patients and the global health system, known as "financial toxicity"[3].
Therefore, the medical community began to explore a new treatment idea-De-escalation. The strategy aims to reduce the toxic and side effects of patients, improve the quality of life, and reduce medical costs without sacrificing the efficacy by reasonably reducing the drug dose or reducing the treatment time.
Targeted drugs act as a "key", specifically targeting specific "locks" on cancer cells (i.e., abnormal proteins produced by mutations of driver genes) to block tumor growth by inhibiting their activity. Different from the chemotherapy model of "killing one thousand enemies at the cost of eight hundred dollars", the efficacy of targeted drugs depends more on such factors as target binding affinity, binding type, target saturation, and the ability to avoid the occurrence of acquired activation pathways in drug-resistant cancer cells than on the concentration of drugs alone[4].
Studies have found that many of the targeted agents require doses that are significantly lower than the doses at which they produce the severe toxicity (i.e., maximum tolerated dose, MTD). Early in drug development, however, MTD was often selected as the recommended dose (RP2D), which may have resulted in systemic "overdose."[5]. A review of 31 new targeted anticancer drugs approved between 2020 and 2023 showed that 20 of them had potential for dose optimization[6]. To this end, the U.S. Food and Drug Administration (FDA) launched the "OPTIMUS Program," which aims to re-evaluate the optimal dose of multiple targeted drugs and promote the shift from the "maximum tolerated dose" to the "optimal therapeutic and safe dose."[7].
Dose reduction is one of the core strategies for treatment degradation. The existing research has explored its feasibility from multiple angles and has achieved some positive results.
Multiple studies have shown that low initial doses or reductions due to side effects of some targeted agents do not affect their long-term efficacy.
· Afatinib: A retrospective study showed that low-dose alfatinib 20mg or 30mg may be comparable to the standard 40mg for patients with advanced NSCLC with EGFR mutations and is more appropriate for Asian patients.[8] Post-hoc analysis of the LUX-Lung 3 and 6 trials also confirmed that progression-free survival (PFS) was not significantly different in patients who had earlier reduced doses due to adverse reactions from those who had not[9].
· Alectinib: J-ALEX study has shown excellent efficacy with 300mg twice daily in Japanese ALK positive patients with PFS data similar to the Asian subgroup with 600mg twice daily in the global Alex study[10,11]. This suggests that lower doses may be equally effective and save up to 50% in different populations.
· Sotorasib: In the Codebreak-100 trial against the KRAS G12C mutation NSCLC, the low-dose 240mg/ day group had similar PFS (5.4 vs 5.6 months) but slightly lower objective response rate (ORR) values (25% vs 33%) compared to the standard 960mg/ day group[12]. This suggests that not all drug reductions are detrimental to efficacy and that more studies are needed to define them. Note: the addition of Fulzerasib and Goselezib(KRAS G12C inhibitors) is recommended for the posterior tier I, and the deletion of Sotorasib (possibly due to efficacy or safety issues).
For drugs with longer half-life, adjusting dosing interval is another innovative way to reduce dose.
· Osimertinib: it has a half-life of about 48 hours[13], theoretically supporting alternate day dosing (EOD). A small-scale real-world study found that the median overall survival (OS) for treated patients who took 80mg every other day was up to 14 months, which was better than that of 40mg taken every other day or every other day[14]. A Phase II clinical trial, NCT05037331, is prospectively evaluating the efficacy and safety of this regimen.
"Boosting" strategy refers to the joint use of a drug (such as CYP3A4 inhibitor) to slow the metabolism of targeted drugs in vivo, so as to maintain the effective blood concentration of targeted drugs while reducing their dosage. The OSIBOOST assay initially demonstrated the potential of Cobicistat to potentiate the efficacy of Osimertinib[15]. This approach offers new avenues to reduce drug costs and toxicity, but is sensitive to potential drug-drug interaction risks.
In addition to reduction, exploration of the optimal treatment duration, and even timely "discontinuation" is another important direction of the demotion strategy, especially in the early adjuvant therapy and the late oligometastatic state.
Among the postoperative adjuvant therapies, the optimal course of treatment has not yet been determined. For example, adjuvant treatment with Osimertinib was 3 years in the ADAURA study[1]Compared to 2 years for Alectinib in the ALINA study[2]. Long-term medication not only brings economic pressure, but also poses great challenge to patients' compliance. A real-world analysis in the UK showed that nearly 40% of patients would discontinue adjuvant Osimertinib treatment[16].
In the future, "adaptive" adjuvant therapy individualized based on dynamic monitoring of minimal residual lesions (MRD) is expected. Detection of circulating tumor DNA(ctDNA) in the blood allows for the determination of whether there are residual cancer cells in the body after surgery. A study in which adjuvant Osimertinib was suspended in patients with undetectable MRD and only 22.7% of patients relapsed during discontinuation suggested that MRD negativity might be a safe "stopping window"[17].
Can systemic therapy be suspended in patients with oligometastases who achieve complete radiographic response after first-line targeted therapy and local consolidation therapy such as radiotherapy? A prospective study gives positive signals[18]. This study included 60 patients with oligometastatic NSCLC who discontinued TKI therapy after achieving complete remission and were monitored by ctDNA.
· The patient's median total time to discontinuation was 9.1 months.
· When ctDNA positivity or disease progression was monitored, restart of TKI treatment resulted in a high response rate of 96%.
· This indicates that a "drug discontinuation-retreatment" strategy (i.e., a "drug vacation") guided by ctDNA is feasible, does not affect the sensitivity to retreatment, and allows patients to be protected from side effects of drugs and financial burden for a period of time.
At present, research on targeted therapy degradation strategy is underway worldwide. The following table summarizes some of the completed and ongoing key clinical trials.
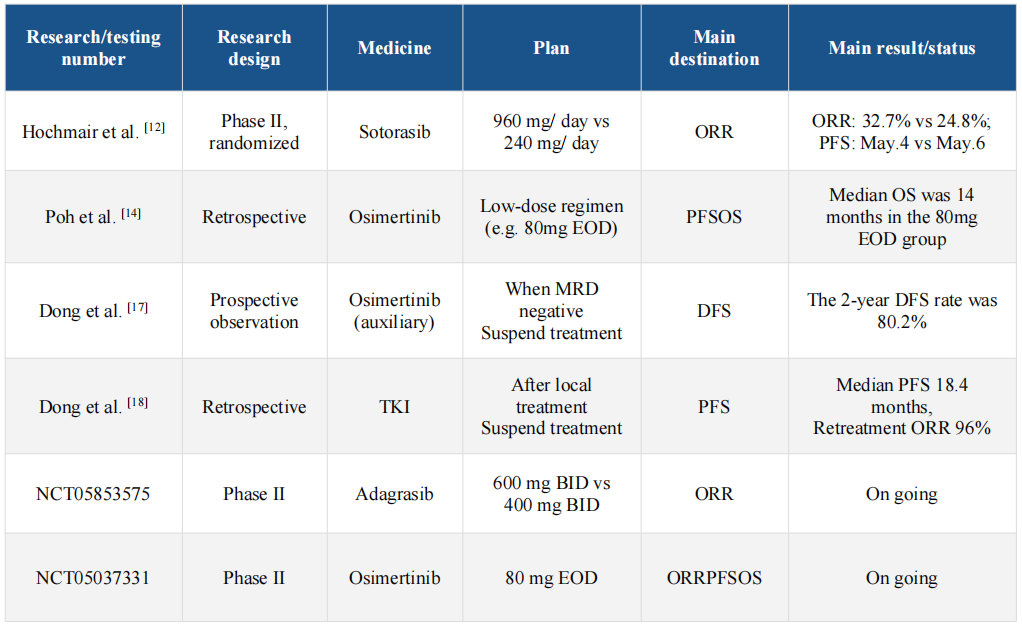
Note: ORR: Objective response rate; PFS: progression-free survival; OS: total survival; DFS: disease-free survival; EOD: Once every other day; BID: twice a day; MRD: minimal residual lesion; TKI: tyrosine kinase inhibitor.
Fig. 1. Therapeutic strategies for de-escalating treatment strategy with targeted therapies.
Despite the promise, the promotion of the downgrade strategy faces many challenges:
· Psychological Disorders: Both patients and physicians may be concerned that "tapering/stopping" equates to suboptimal treatment, which can affect disease control.
· Resistance risk: Long-term data validation is still needed to determine whether intermittent treatment will accelerate the emergence of resistance mechanisms.
· Missing criteria: There is currently a lack of standardized screening criteria to determine which patients are eligible for demotion and a lack of mature biomarkers to guide decision-making.
In the future, more well-designed non-inferiority clinical trials and the inclusion of comprehensive endpoints such as quality of life, cost-effectiveness and patient reporting outcome (PROs) are needed to provide a higher level of evidence for the demotion strategy.
The philosophy of "subtraction" in targeted therapy represents a conceptual shift from "maximizing therapy" to "optimizing therapy". Available data indicate that a dose reduction or course reduction strategy is feasible and has potential in the field of NSCLC. This is not only expected to reduce the physical and economic burden on patients, but also push the global cancer treatment towards a more efficient, fair and sustainable future. Of course, the realization of this goal requires the joint efforts and wisdom of scientific research, clinical and regulatory institutions as well as the patient group.
1. N Engl J Med 2020; 383(18):1711–23.
2. N Engl J Med apr. 2024; 390(14):1265–76.
3. J Clin Oncol giu. 2023; 41(16): 3051–8.
4. Cancers mar. 2020; 12(3):731.
5. Lancet Oncol ago. 2024; 25(8):e340–51.
6. N Engl J Med ott. 2021; 385(16):1445–7.
7. Lung Cancer gen. 2020; 139:195–9.
8. J Thorac Oncol lug. 2019; 14(7):1233–43.
9. O. of the Commissioner, Project Optimus, FDA, giu. 2024.
10. Transl. Lung Cancer Res. feb. 2024; 13(2):307–20.
11. J. Eur. Soc. Med. Oncol. nov. 2016; 27(11):2103–10.
12. Eur J Cancer set. 2024; 208:114204.
13. Lung Cancer set. 2022; 171:97–102.
14. Clin Pharmacol Drug Dev feb. 2019; 8(2):198–207.
15. Lung Cancer Res. lug. 2024; 13(7).
16. Ann Oncol set. 2024; 35: S782–3.
17. JTO Clin. Res. Rep. nov. 2024.
18. JAMA Oncol lug. 2024; 10(7):932.